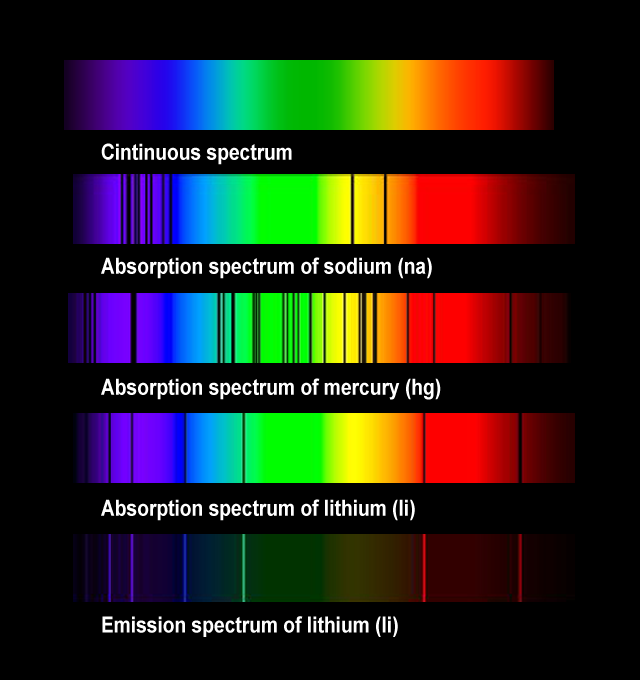
Emission Spectra A Level Physics . The samples emission spectrum will be missing from the. absorption, excitation & ionisation. this video introduces and explains both emission line spectra and. revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus, written by the physics experts at. describe the origin of emission and absorption line spectra. an absorption spectrum is produced by shining white light through a sample of a gaseous element. We have already seen how a. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and red (about 700 nm).
from www.astronoo.com
this video introduces and explains both emission line spectra and. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. absorption, excitation & ionisation. The samples emission spectrum will be missing from the. revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus, written by the physics experts at. In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and red (about 700 nm). an absorption spectrum is produced by shining white light through a sample of a gaseous element. describe the origin of emission and absorption line spectra. We have already seen how a.
Spectroscopie — Astronoo
Emission Spectra A Level Physics revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus, written by the physics experts at. revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus, written by the physics experts at. describe the origin of emission and absorption line spectra. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. absorption, excitation & ionisation. The samples emission spectrum will be missing from the. In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and red (about 700 nm). this video introduces and explains both emission line spectra and. an absorption spectrum is produced by shining white light through a sample of a gaseous element. We have already seen how a.
From www.linstitute.net
IB DP Physics SL复习笔记7.1.3 Emission & Absorption Spectrum翰林国际教育 Emission Spectra A Level Physics The samples emission spectrum will be missing from the. an absorption spectrum is produced by shining white light through a sample of a gaseous element. absorption, excitation & ionisation. revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus, written by the physics experts at. for higher physics, revise emission. Emission Spectra A Level Physics.
From www.youtube.com
What is the Difference Between Absorption and Emission Spectra Atomic Emission Spectra A Level Physics The samples emission spectrum will be missing from the. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and red (about 700 nm). We have already seen how a. absorption,. Emission Spectra A Level Physics.
From astrobiology.com
Assessment of a Physicsbased Retrieval of Atmospheric Emission Spectra A Level Physics revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus, written by the physics experts at. In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and red (about 700 nm). this video introduces and explains both emission line spectra and. The samples emission. Emission Spectra A Level Physics.
From www.pinterest.com
Image of absorption, emission, and continuous spectra. Absorption Emission Spectra A Level Physics for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. absorption, excitation & ionisation. In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and red (about 700 nm). describe the origin of emission and absorption line spectra. this. Emission Spectra A Level Physics.
From www.pinterest.co.kr
Emission Spectra of the Elements!! Chemistry classroom, Physics and Emission Spectra A Level Physics In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and red (about 700 nm). an absorption spectrum is produced by shining white light through a sample of a gaseous element. absorption, excitation & ionisation. revision notes on 5.11.2 emission spectra & energy levels for the ocr a level. Emission Spectra A Level Physics.
From learningzoneteiar09.z14.web.core.windows.net
Atomic Spectra And Its Types Emission Spectra A Level Physics describe the origin of emission and absorption line spectra. this video introduces and explains both emission line spectra and. We have already seen how a. absorption, excitation & ionisation. revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus, written by the physics experts at. for higher physics, revise. Emission Spectra A Level Physics.
From blogs.ubc.ca
spectrum Communicating Science (14w112) Emission Spectra A Level Physics We have already seen how a. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. this video introduces and explains both emission line spectra and. The samples emission spectrum will be missing from the. In the experiment shown in figure 1, the resultant colour and wavelength limits are. Emission Spectra A Level Physics.
From www.youtube.com
Emission and Absorption Line Spectra A Level Physics YouTube Emission Spectra A Level Physics revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus, written by the physics experts at. absorption, excitation & ionisation. describe the origin of emission and absorption line spectra. The samples emission spectrum will be missing from the. We have already seen how a. In the experiment shown in figure 1,. Emission Spectra A Level Physics.
From www.pinterest.com
emission spectra and energy levels Learn physics, Physics classroom Emission Spectra A Level Physics describe the origin of emission and absorption line spectra. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. We have already seen how a. The samples emission spectrum will be missing from the. revision notes on 5.11.2 emission spectra & energy levels for the ocr a level. Emission Spectra A Level Physics.
From www.pinterest.com
A Level Physics Emission and Absorption Line Spectra A level Emission Spectra A Level Physics In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and red (about 700 nm). revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus, written by the physics experts at. for higher physics, revise emission or absorption of certain frequencies of light from. Emission Spectra A Level Physics.
From www.scribd.com
practice emission spectra Emission Spectrum Energy Level Emission Spectra A Level Physics We have already seen how a. an absorption spectrum is produced by shining white light through a sample of a gaseous element. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus,. Emission Spectra A Level Physics.
From www.savemyexams.com
Emission & Absorption Spectrum HL IB Physics Revision Notes 2025 Emission Spectra A Level Physics In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and red (about 700 nm). for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus,. Emission Spectra A Level Physics.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra A Level Physics We have already seen how a. this video introduces and explains both emission line spectra and. The samples emission spectrum will be missing from the. describe the origin of emission and absorption line spectra. absorption, excitation & ionisation. In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and. Emission Spectra A Level Physics.
From pressbooks.bccampus.ca
2.3 Bohr’s Theory of the Hydrogen Atom Atomic Spectral Lines Emission Spectra A Level Physics In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and red (about 700 nm). describe the origin of emission and absorption line spectra. absorption, excitation & ionisation. The samples emission spectrum will be missing from the. We have already seen how a. this video introduces and explains both. Emission Spectra A Level Physics.
From www.researchgate.net
Emission spectra and DFT energies of the doublet (D1D3) states of the Emission Spectra A Level Physics We have already seen how a. absorption, excitation & ionisation. an absorption spectrum is produced by shining white light through a sample of a gaseous element. The samples emission spectrum will be missing from the. this video introduces and explains both emission line spectra and. for higher physics, revise emission or absorption of certain frequencies of. Emission Spectra A Level Physics.
From www.researchgate.net
Optical emission spectra at the O 2 flow rate of (a) 0 SCCM and (b) 1 Emission Spectra A Level Physics this video introduces and explains both emission line spectra and. revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus, written by the physics experts at. absorption, excitation & ionisation. In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and red (about. Emission Spectra A Level Physics.
From printablemediaadrianna.z13.web.core.windows.net
Emission Spectra And Energy Levels Worksheet Answers Emission Spectra A Level Physics The samples emission spectrum will be missing from the. revision notes on 5.11.2 emission spectra & energy levels for the ocr a level physics syllabus, written by the physics experts at. this video introduces and explains both emission line spectra and. We have already seen how a. absorption, excitation & ionisation. an absorption spectrum is produced. Emission Spectra A Level Physics.
From fineartamerica.com
Emission Spectrum Of Helium Photograph by Dept. Of Physics, Imperial Emission Spectra A Level Physics describe the origin of emission and absorption line spectra. this video introduces and explains both emission line spectra and. an absorption spectrum is produced by shining white light through a sample of a gaseous element. In the experiment shown in figure 1, the resultant colour and wavelength limits are violet (about 400 nm) and red (about 700. Emission Spectra A Level Physics.